|
Современная
физика.
FEATURES OF KINETICS OF THERMAL DESTRUCTION OF HMX
Burov Yuri*, Dubikhin Valery*, Kovalchukova Olga**
* Institute of Problems of Chemical Physics, RAS, 142432 Chernogolovka, RF
** Peoples' Friendship University of Russia , 117198, Moscow , RF
E-mail: yuburov@icp.ac.ru
Abstract :
Features of kinetics of thermal decomposition of HMX dealing with polymorphous transitions and auto-catalysis in a solid state were studied.
Keywords: thermal decomposition, HMX, polymorphic modification, polymorphic transitions, autocatalysis
INTRODUCTION
Existence of 4 polymorphous modifications of HMX and auto-catalysis in condensed state makes kinetics of their decomposition extremely complicated.
Properties of different modifications of HMX are clearly described in [1] . The 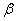 -modification is stable in the temperature interval from 22 till 115 0 С , the -modification is stable in the temperature interval from 22 till 115 0 С , the 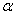 -modification exists from 115 till 156.5 0 С . The -modification exists from 115 till 156.5 0 С . The 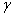 -modification exists at 156.5, as far as -modification exists at 156.5, as far as 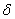 -modification – from 156.5 0 С till the melting point. In pure solids the transitions are slow down. This fact allows to study the bthermal destruction of HMX out of areas of their thermal stability (in the meta-stable state). -modification – from 156.5 0 С till the melting point. In pure solids the transitions are slow down. This fact allows to study the bthermal destruction of HMX out of areas of their thermal stability (in the meta-stable state).
EXPERIMENTAL
The thermal destruction of HMX was studied manometrically under static conditions. Aero-thermostats allowed to heat glacial Burdon's vessels within 5 – 6 min. The rate constants were calculated using the 1-st rate equation on the linear part of the graph, the final gas-evolution was considered as 600 cm 2 ·g -1 [2] .
Synthesis of different modifications of HMX was performed according to [1] .
The 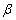 -modification of HMX was obtained from the technical -modification of HMX was obtained from the technical 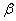 - HMX by its re-crystallization from acetone and further vacuum-drying at 50 0 С during 6 h. Small amount of volatile impurities allowed to perform experiments at maximal rates of filling of the reactional vessel. The - HMX by its re-crystallization from acetone and further vacuum-drying at 50 0 С during 6 h. Small amount of volatile impurities allowed to perform experiments at maximal rates of filling of the reactional vessel. The 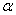 -modification of HMX was obtained by dissolution of the -modification of HMX was obtained by dissolution of the 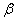 -modification in boiling 60 per-cent nitric acid and further cooling without stirring. The obtained crystals were washed by water, and dried under vacuum during 6 h. The presence of impurities of solvents didn't allow to perform experiment at -modification in boiling 60 per-cent nitric acid and further cooling without stirring. The obtained crystals were washed by water, and dried under vacuum during 6 h. The presence of impurities of solvents didn't allow to perform experiment at 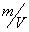 greater that 0.1 g·cm -3 . Heating of the greater that 0.1 g·cm -3 . Heating of the 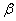 -modification of the HMX higher than 180 0 С leaded to formation of the -modification of the HMX higher than 180 0 С leaded to formation of the 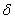 -modification. The polymorphous composition of the HMX was detected by DSK. -modification. The polymorphous composition of the HMX was detected by DSK.
The 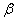 -modification of HMX was studied in the temperature interval 130 – 250 0 С , and three intervals were found. -modification of HMX was studied in the temperature interval 130 – 250 0 С , and three intervals were found.
1. 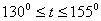 . The kinetics curve consists of the initial fragment, which is followed by the acceleration stage (fig.1). The rate constant of the decomposition of the . The kinetics curve consists of the initial fragment, which is followed by the acceleration stage (fig.1). The rate constant of the decomposition of the 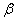 -modification -modification 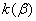 was determined using the initial linear part of the graph. After the acceleration, rate constant of the decomposition of the was determined using the initial linear part of the graph. After the acceleration, rate constant of the decomposition of the 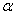 -modification obtained after the polymorphous transition of the -modification obtained after the polymorphous transition of the 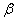 -modification, -modification, 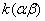 , was determined , was determined
2. 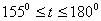 . The time of the polymorphous transition is comparable with the time of the duration of the experiment. The kinetic curve has the initial linear part dealing with decomposition of the . The time of the polymorphous transition is comparable with the time of the duration of the experiment. The kinetic curve has the initial linear part dealing with decomposition of the 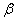 -modification of HMX, then the fragment described by the 1-st order equation which is related to the polymorphous transition. After it the linear fragment described as the decomposition of the -modification of HMX, then the fragment described by the 1-st order equation which is related to the polymorphous transition. After it the linear fragment described as the decomposition of the 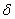 -modification takes place. It is well seen on the experiment of thermal decomposition of the technical HMX. The -modification takes place. It is well seen on the experiment of thermal decomposition of the technical HMX. The 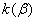 was determined from the initial linear part of the graph. was determined from the initial linear part of the graph.
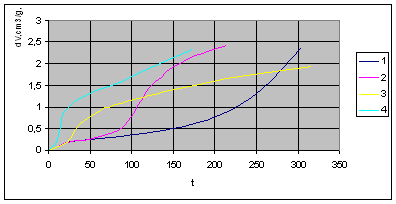
3. 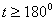 . The . The 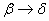 transition takes place within the time of heating of the sample. The proceeding of the reaction during the polymorphous transition is proved only by rather high starting gas-evolution. The value of transition takes place within the time of heating of the sample. The proceeding of the reaction during the polymorphous transition is proved only by rather high starting gas-evolution. The value of 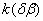 was determined from the initial linear part of the curve. was determined from the initial linear part of the curve.
Figure 1 . The thermal decomposition of HMX at the polymorphic transition at the temperatures: 1 – 165 0 C, 2 – 170 0 C, 3 – 175 0 C, 4 – 180 0 C
The destruction of the 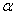 -modification of HMX was studied in the temperature interval from 140 to 250 0 С . In the interval 140 – 180 0 С , the values of -modification of HMX was studied in the temperature interval from 140 to 250 0 С . In the interval 140 – 180 0 С , the values of 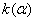 were determined using the initial part of the dependence. At 200 – 250 0 С , the were determined using the initial part of the dependence. At 200 – 250 0 С , the 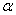 -modification of HMX was transmitted to the -modification of HMX was transmitted to the 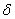 -modification, and the -modification, and the 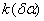 was determined. was determined.
To determine the rate constants of the thermal decomposition of a single sample in various polymorphic modifications, the 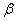 - HMX was transmitted to the - HMX was transmitted to the 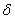 -HMX by the temperature evaluation till 220 0 С for 0.5 h. Then the temperature was quickly decreased to 170, 150, 140, and 130 0 С . The quick cooling didn't allow the transition of the sample to the -HMX by the temperature evaluation till 220 0 С for 0.5 h. Then the temperature was quickly decreased to 170, 150, 140, and 130 0 С . The quick cooling didn't allow the transition of the sample to the 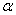 -modification, and the value of -modification, and the value of 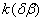 could be determined in the interval 170 – 140 0 С , as far as could be determined in the interval 170 – 140 0 С , as far as 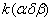 was determined at 130 0 С . In case if was determined at 130 0 С . In case if 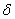 - HMX was kept under room temperature for 2 days, it transited to the - HMX was kept under room temperature for 2 days, it transited to the 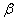 -modification, and the value of -modification, and the value of 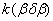 was determined at 140 and 150 0 С . was determined at 140 and 150 0 С .
To study the influence of sizes of particles, the crystals of HMX underwent grinding, compressing and re-crystallization.
The accuracy of measuring and maintaining of the temperature was 0.1 0 С . The accuracy of the rate constants measuring in majority of cases was 10 per cents, and for 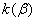 it decreased to 100 per cents because of the necessity to measure small pressure differences within long time intervals (the durance of one experiment reached one month). it decreased to 100 per cents because of the necessity to measure small pressure differences within long time intervals (the durance of one experiment reached one month).
RESULTS AND DISCUSSION
The most important results of the above experiments are presented on fig. 2 and table 1.
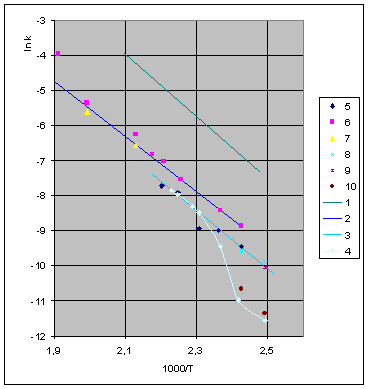
Figure 2. Arrhenius' dependence of the rate constant of the thermal decomposition of HMX: 1 – decay in the gase phase [6] , decay in the solid state; 2 - 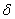 - HMX, 3 - - HMX, 3 - 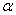 - HMX, 4 - - HMX, 4 - 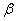 - HMX, 5 - - HMX, 5 - 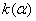 , 6 - , 6 - 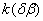 , 7 - , 7 - 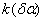 , 8 - , 8 - 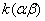 , 9 - , 9 - 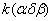 , 10 - , 10 - 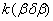 . .
The 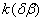 value is close to those measured in [3, 4] but the temperature dependence doesn't change in the temperature range 140 – 230 0 С . The deviation from the Arrhenius' dependence at 250 0 С can be described as following. Because of a high rate of decomposition, the measurements were performed at a small value is close to those measured in [3, 4] but the temperature dependence doesn't change in the temperature range 140 – 230 0 С . The deviation from the Arrhenius' dependence at 250 0 С can be described as following. Because of a high rate of decomposition, the measurements were performed at a small 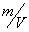 (about 10 -3 g·cm -3 ) and at a high (up to 10 per cent) decomposition deepness. (about 10 -3 g·cm -3 ) and at a high (up to 10 per cent) decomposition deepness.
Table 1. Rate constants of the thermal decomposition of HMX
T 0 C |
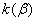
|
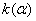
|
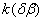
|
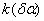
|

|
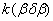
|
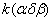
|
130 |
2.81·10 -12 |
- |
- |
- |
1.5·10 -12 |
5.6·10 -12 |
8.9·10 -11 |
140 |
1.12·10 -11 |
3.2·10 -10 |
1.77·10 -9 |
- |
1.33·10 -9 |
2.8·10 -11 |
- |
150 |
4.36·10 -10 |
1.08·10 -9 |
3.98·10 -9 |
- |
- |
- |
- |
160 |
3.8·10 -9 |
1.1·10 -9 |
- |
- |
- |
- |
- |
170 |
1.13·10 -8 |
1.05·10 -8 |
3.8·10 -8 |
- |
- |
- |
- |
180 |
- |
2.39·10 -8 |
7.90·10 -8 |
- |
- |
- |
- |
190 |
- |
- |
1.7·10 -7 |
- |
- |
- |
- |
200 |
- |
- |
4.97·10 -7 |
3.26·10 -7 |
- |
- |
- |
220 |
- |
- |
2.04·10 -6 |
- |
- |
- |
- |
230 |
- |
- |
4.7·10 -6 |
2.25·10 -6 |
- |
- |
- |
250 |
- |
- |
1.52·10 -4 |
1.38·10 -4 |
- |
- |
- |
The rate constant 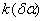 is a little bit less then is a little bit less then 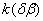 but this difference can be affected by pre-preparation methods (re-crystallization from different solvents, grinding, compression and so on). The but this difference can be affected by pre-preparation methods (re-crystallization from different solvents, grinding, compression and so on). The 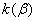 values measured at 150 and 170 0 С are in a good accordance with the constants measured in [4] but the temperature dependence significantly differs from the Arrhenius' one. The reason of a quick acceleration of the reaction near 150 0 C can not be the transition of the substance into the values measured at 150 and 170 0 С are in a good accordance with the constants measured in [4] but the temperature dependence significantly differs from the Arrhenius' one. The reason of a quick acceleration of the reaction near 150 0 C can not be the transition of the substance into the 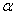 -modification. The transition -modification. The transition 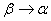 at 150 0 С is not detected by DTA within 40 h. The decomposition rate constant determined at 140 0 С after the experiment at 150 0 C was found to be at 150 0 С is not detected by DTA within 40 h. The decomposition rate constant determined at 140 0 С after the experiment at 150 0 C was found to be 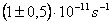 and correlates with and correlates with 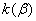 . At 160 – 175 0 С , the rate constant of decomposition of . At 160 – 175 0 С , the rate constant of decomposition of 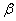 - HMX equals to that of - HMX equals to that of 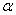 - HMX. - HMX. 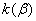 = = 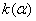 . This abnormal behavior of . This abnormal behavior of 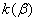 near the temperature of the polymorphic transition (156.5 0 С may be related with the increase of the amplitude of thermal oscillations of molecules near the point of polymorphic transition [5] . near the temperature of the polymorphic transition (156.5 0 С may be related with the increase of the amplitude of thermal oscillations of molecules near the point of polymorphic transition [5] .
Autocatalysis of HMX is of a complex nature. It can be the one of gaseous products with the submelting of a solid initial substance by products of its decomposition. But at early stages with no liquid phase there is an autocatalysis in a solid state. It was detected visually that HMX crystals change the color to yellow (or brown) while decomposing. The quote of yellow crystals increases with the increase of the deepness of decomposition. During the experiment, a HMX-sample underwent a partial decomposition. Then the yellow crystals were separated from the white ones, and the experiment continued for both of them. It appeared that the rate of decomposition of white crystals is the same that the initial rate of decomposition as far as for the yellow crystals it equaled the rate of decomposition at the moment the first part of the experiment was stopped. Thus, the role of autocatalysis was visually shown. Every pre-treatment of the sample followed by the crystal size decrease diminish the size of the area of yellowshing of crystals, and thus decrease the rate of decomposition. Such a dependence is an abnormal one as far as usually the rate of decomposition increases with the decrease in particle sizes because of the increase of the degree of the surface decomposition versus the reaction in the volume [6] .
The polymorphous transitions together with the abnormal dependence of the rate of thermal decomposition on the crystal sizes make kinetics of thermal decomposition of HMX complicated and not clear as it depends not only on the temperature but on the history of the sample, its pre-treatment and rate of heating.
REFERENCES
[1]. Орлова Е.Ю., Орлова Н.А., Жилин В.Ф. и др. Октоген – термостойкое взрывчатое вещество. М.: Недра, 1975.
[2]. Максимов Ю.Я. В кн.: Теория взрывчатых веществ. Труды МХТИ им. Д.И. Менделеева, вып. 53 . М.: Высшая школа, 1967, с. 73.
[3]. Медведев А.И., Сакович Г.В., Константинов В.В. В кн .: Совещание по кинетике и механизму химических реакций в твердом теле. Тезисы докладов. Часть I . Новосибирск, 1977, с. 163.
[4]. Беляева М.С., Клименко Г.К., Бабайцева Л.И. Столяров П.Н. В кн .: Химическая физика процессов горения и взрыва. Кинетика химических реакций. Черноголовка, 1977, с. 47.
[5] Бокштейн Б.С., Бокштейн С.З., Жуховицкий А.А. Термодинамика и кинетика диффузии в твердых телах. М.,1974 .
[6]. Bon S. In Chemistry of the Solid State , Ed. W. E. Garner, Butterworths, London , 1955. P. 335.
Fatal error: Uncaught Error: Call to undefined function set_magic_quotes_runtime() in /www/htdocs/1dbcf2b3552b065fc49d8747114db86c/sape.php:262
Stack trace:
#0 /www/htdocs/1dbcf2b3552b065fc49d8747114db86c/sape.php(343): SAPE_base->_read('/www/htdocs/1db...')
#1 /www/htdocs/1dbcf2b3552b065fc49d8747114db86c/sape.php(418): SAPE_base->load_data()
#2 /www/htdocs/links.html(7): SAPE_client->SAPE_client()
#3 /www/htdocs/modern_physics/z1.html(266): include('/www/htdocs/lin...')
#4 {main}
thrown in /www/htdocs/1dbcf2b3552b065fc49d8747114db86c/sape.php on line 262
| 
